Most people associate nature with beautiful and well-ordered systems, but this is not always the case. A new groundbreaking study of the growth hormone receptor, a key regulator of the human body, shows that it has such a disordered structure that researchers have difficulty believing it. The results open up new avenues towards fully understanding the biology of the receptor and may eventually help in developing new strategies to treat insufficient growth in height and hormone deficiencies.
When you open a door, most people probably see a simple and well-defined lock into which you insert a key. Receptors are the doors of cells – dividing the internal environment of the cell from the surrounding environment. The doors open and close depending on what the cell and the body as a whole need. However, the locks that open and enable signals and molecules to pass through are often far more complicated and disordered than researchers previously thought. Imbalances in the receptors can strongly affect health and disease, and the receptors are therefore also key to drug development.
“Because of their small size and great structural chaos, proteins such as the human growth hormone receptor prevent us from using conventional methods to determine their structure. However, we have finally succeeded in combining methods to determine for the first time the structure of this type of full-length membrane protein embedded in a realistic membrane environment. The result is remarkable, because although the exterior of the door lock is well ordered, the interior is a spaghetti-like chaos we do not fully comprehend yet that strongly influences how we understand biology and therefore also how we treat people with hormonal disorders in the future,” explains Birthe B. Kragelund, Professor and Group Leader, Novo Nordisk Foundation Challenge Center REPIN, Department of Biology, University of Copenhagen.
Orphan proteins of structural biology
The study focused on the human growth hormone receptor. This receptor is activated when growth hormone binds to it, and turning it on or off strongly affects many processes such as the growth of muscles and bones, metabolism and especially the immune system. Even though artificial human growth hormone has been produced for more than 50 years, researchers had not been able to determine what the receptor in the cells looks like in full size.
“There are myriad crystal structures of the part of the receptor outside the cell – the part to which the hormone binds. However, the structures are typically of the receptor together with the hormone or similar substances that bind to it. These structures have provided very important knowledge about how growth hormone binds to the receptor and how drugs could be developed that prevent or amplify the receptor’s signals. Nevertheless, the exterior only solves half the riddle. The other half is located inside the membrane,” says Birthe B. Kragelund.
Determining the structure of the receptor was a major methodological challenge. It has a well-ordered external domain, a membrane-embedded helix structure and a disordered internal domain without structure. Its small size (70 kilodaltons) and the extensive structural chaos in most of it had prevented researchers from determining the structure using conventional methods – until now.
“The mapping of the human genome two decades ago showed that more than 30% of our proteome is intrinsically disordered proteins – proteins without a fixed structural form. Since they have many dynamic forms, they cannot be crystallized or photographed by electron microscopy. This has posed a methodological challenge, and receptors such as the human growth hormone receptor have thus been orphaned in structural biology. Determining the structures of these proteins and thereby understanding them better has required researchers to work extremely integratively – much more than before,” explains Birthe B. Kragelund.
A springboard
Receptors are generally complex proteins, and understanding their full structure has therefore required interdisciplinary research collaboration that unites seven research groups from Denmark, Canada, Germany and Sweden, specializing in very different disciplines such as yeast expression, mass spectrometry, protein chemistry, biophysics, X-ray crystallography, molecular dynamics and modelling, nuclear magnetic resonance (NMR) spectroscopy and neutron and small-angle X-ray scattering. The researchers studied the intact receptor in its environment of a lipid membrane for the first time.
“For complex proteins such as the human growth hormone receptor, we cannot determine its structure by using any of the classical experimental methods such as crystallography, NMR or cryoelectron microscopy. In short, the human growth hormone receptor is far too flexible for crystallography and cryoelectron microscopy but far too large for in-depth NMR analysis,” says co-author Lise Arleth, Professor in Experimental Biophysics, Niels Bohr Institute and Associate Dean for Research, Faculty of Science, University of Copenhagen, adding: “Small-angle neutron and X-ray scattering can manage the entire protein structure under close to natural conditions but unfortunately provides far less detailed information than the classic technologies and therefore cannot be used independently.”
Lis Arleth also says: “So far, we have used the divide-and-conquer approach, studying the individual domains or regions separately and then integrating the individual pieces of information into a single image – like a patchwork quilt. One important breakthrough in this work was stabilizing the human growth hormone receptor molecule in a nanodisc, which contains a small slice of lipid membrane. Thus, we could use small-angle scattering to see the entire contour of the total dynamic molecule while in the membrane. This then gave us an overall framework into which we could add other bits of experimental information through molecular dynamics and protein modelling.”
“Each individual measurement did not provide a full picture, but overall we created the first structure ever of a full-length membrane protein embedded in a realistic membrane environment – containing a large disordered chain. This will be a springboard to improving understanding of the human growth hormone receptor and will transform how we envision the biology and cellular signalling of these disordered proteins,” adds Birthe B. Kragelund.
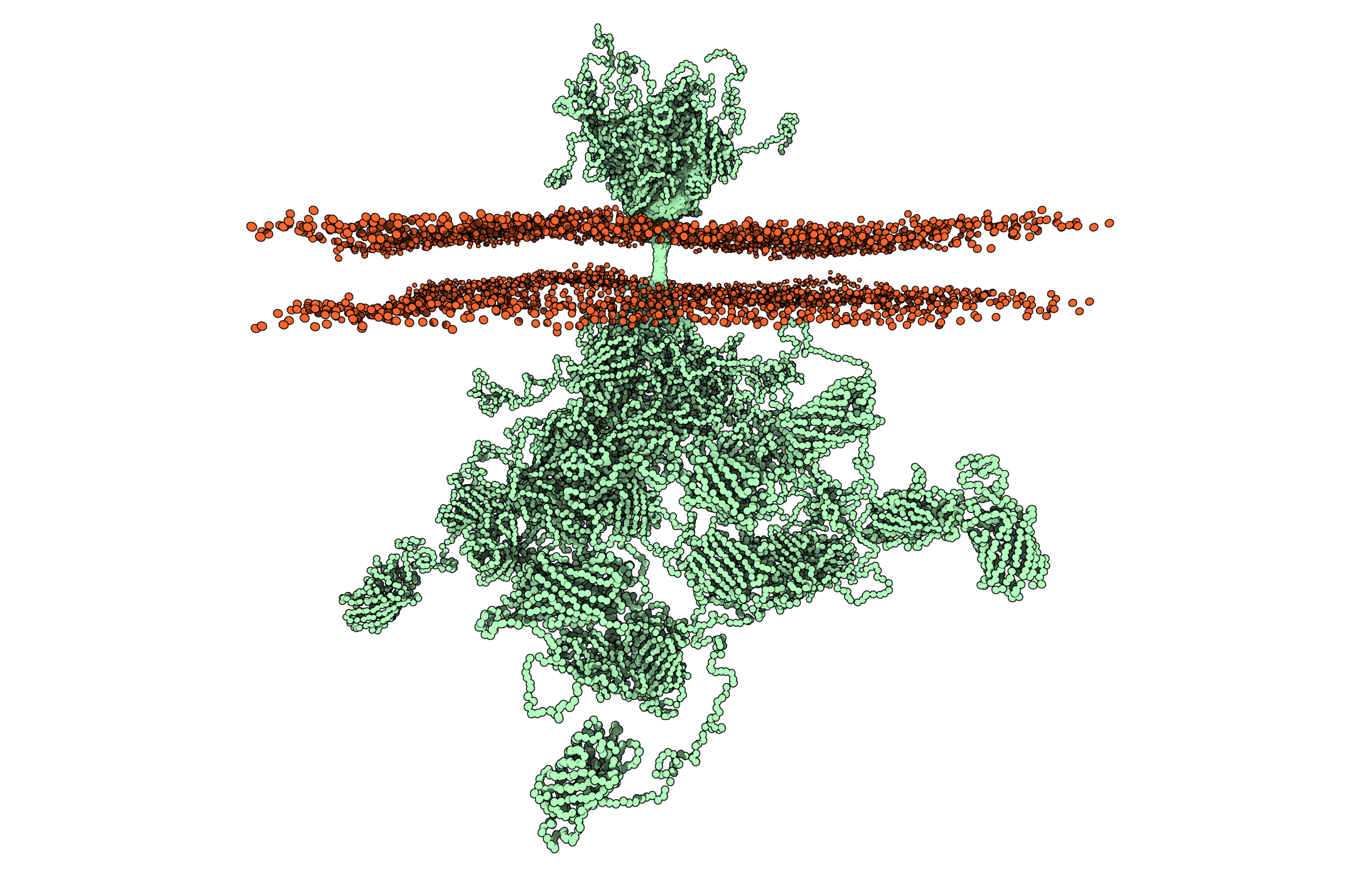
Chaos is the new normal
The new collaboration thus shows how integrating experimental data from several techniques by computer can arrive at structures of proteins with long, disordered regions – a widespread phenomenon in biology. About one fifth of our proteins have both order and disorder, and some have very long disordered domains.
“We found that the part of the receptor outside the cell we had examined so far is really just the tip of a giant iceberg. When the growth hormone binds to the receptor, it activates several intracellular signalling cascades. Nevertheless, what happens within the cell walls, when the hormone binds to the receptor, has been extremely difficult to determine,” explains another co-author, Kresten Lindorff-Larsen, Group Leader, BRAINSTRUC Lundbeck Foundation initiative and Professor, Kaj Ulrik Linderstrøm-Lang Centre for Protein Science, Department of Biology, University of Copenhagen.
For the first time, the researchers now have a real image of what the receptor looks like – also inside – but naturally, several new interesting questions arise. In particular, the disordered inner part is still mysterious.
“We may have been side-tracked by the fact that the structures that were historically first determined experimentally were the most well-ordered. Perhaps disorder and chaos is actually the normal state,” says Kresten Lindorff-Larsen.
System brakes
The new result is also the first experimental model of the group of full-length cytokine receptors embedded in a membrane. In recent years, these receptors have received much attention because a lack has been linked to debilitating immunodeficiency.
“Nevertheless, we do not yet know whether the human growth hormone receptor is a representative model for this whole group of receptors. We need to understand how this simple binding of a substance on the well-ordered exterior can result in the correct signal from the spaghetti-like chaos on the inside of the cell. How the binding of the hormone can affect the movements down through the membrane and perhaps change the properties of the disordered part – that is, how communication takes place through the disorder – is enigmatic and unbelievably fascinating,” explains Birthe B. Kragelund.
If the researchers learn to understand and sort the chaos and thus distinguish the ensemble of signals on the inside, this can affect the treatment of people with diseases such as various types of cancer. Similarly to how growth hormone supplements changed the situation for growth, such as those Lionel Messi was given as a child, a situation is emerging with opportunities to discover all the other functions of the receptor. The new knowledge can enable completely new treatment strategies in the long term.
“The typical methods of disease treatment have either put a brake into the system to prevent the receptor from forming its signalling cascade or given the hormone as a supplement to activate the receptor. Now we have a much better platform we can use to study the individual signalling pathways, and in the long term this will enable them to be turned on or off one at a time,” concludes Birthe B. Kragelund.
