Microglia are the brain’s garbage collectors. Studying them for a lifetime probably sounds reasonably boring to most people. However, for Marco Prinz, the world’s most boring brain cells were his path into the incredible world of brain research. For almost 25 years, his research has revealed that microglia are much more than garbage collectors. They are the key to the brain’s immune system and thus to understanding autoimmune brain disorders such as Alzheimer’s and multiple sclerosis. Microglia may be the key to treating people who have these diseases. Additionally, microglia also control many other key functions of the brain. For his pioneering studies, Marco Prinz is receiving the 2021 Novo Nordisk Prize.
The blood–brain barrier is established prenatally to prevent influx of immune cells at a later time. Thus, the brain hosts its own immune system with a key actor called microglia. In addition, the microglia play a central role in both monitoring and defending the brain and maintaining its normal functions. At the beginning of his career, Marco Prinz became fascinated by these cells, which received almost no attention in the mid-1990s and were primarily thought to be the brain’s garbage collectors.
“Most people found them boring, because they were thought to simply remove waste during illness. Our research has shown that their function extends far beyond this role and that the development of microglia dates back to a remarkably short period during fetal development. Microglia are key in maintaining the normal function of the brain, and their failure can therefore also lead to several very serious brain disorders,” explains Professor Dr. Marco Prinz, Director at the Institute of Neuropathology, Medical Center, University of Freiburg, Germany.
Exotic research
Microglial cells were not at the top of Marco Prinz’s wish list when he completed his medical studies at Humboldt University (Charité) in Berlin in 1996. The brain had always fascinated him, but with the still rather rudimentary conditions in eastern Germany at that time, the opportunities for research were very limited. However, he got the chance to investigate brain cell staining to study neuronal growth among children with Down’s syndrome.
“We used very old-fashioned silver staining techniques, but my studies with neuroanatomist Eveline Schulz made me want to explore the brain more, and this led me to choose research rather than the medical path.”
This was just after the Berlin Wall fell, and in an attempt to integrate eastern and western Germany, a decision was made to build new research institutes in eastern Germany. One was the Max Delbrück Center for Molecular Medicine (MDC) in Berlin.
“I asked around a bit: what is your research field? One researcher worked with stroke and another worked with encephalitis. And this guy who works on encephalitis then said he was collaborating with someone else, so I thought, ‘Okay, I’ll try my luck there and see what he does in the lab’.”
Most brain researchers worked with neurons and the signals between them, but biochemist Uwe-Karsten Hanisch did not. He believed that inflammation of the brain is essentially mediated by a special type of cells called microglia.
At the time, they were thought to be a kind of garbage collector that simply cleaned up after brain infections. Very few people were working with these very special immune cells in the brain. This was a really exotic research field, and yes, by that time, microglia were probably the most boring cells in the brain.The trigger release
Despite Marco Prinz’s medical background and sparse education in traditional research, Uwe-Karsten Hanisch took Marco Prinz under his wings as a postdoctoral fellow. This turned out to be an excellent choice for both of them, and soon they produced a steady stream of articles on microglia and interleukins, the immune system’s tiny signalling molecules. However, brain researchers showed little interest because their focus was elsewhere.
In 1997, Stanley Prusiner from the United States received the Nobel Prize for his explanation of the cause of bovine spongiform encephalopathy (mad cow disease) and the human counterpart Creutzfeldt-Jakob disease. He coined the term prion from the words proteinaceous and infectious to explain how the misfolding of proteins caused the infection in diseases. Adriano Aguzzi from the University Hospital of Zurich, who took Prinz in as his postdoctoral fellow was also one of the world-leading experts on prion diseases during those days.
“The people I met here were the outstanding scientists and teachers: Adriano Aguzzi, a natural and driven neuropathologist ; Charles Weissmann, who pioneered molecular genetics, cloned type I interferons and was also one of the founders of Biogen, worked in a nearby laboratory; and then there was Rolf Zinkernagel, who had actually won the Nobel Prize in Physiology or Medicine just a few years before. They were really demanding and brilliant scientists from whom I learned my basic understanding of molecular medicine."
During this time, Marco Prinz achieved his first major scientific advances, which contributed to understanding a mechanism for transferring prions from the immune system to the nervous system. The results were published in major journals such as Proceedings of the National Academy of Sciences of the United States of America in 2002 and Nature in 2003 and other leading journals.
In hindsight, I clearly had to be there to move on to bigger things. It was a really intense time, and learning how to conduct experiments and think about science was important. This was something that I really learned together with these incredibly serious individualsEven though Marco Prinz had to put aside the microglia while he was in Zurich, they continued to turn up. Indeed, growing evidence showed that microglia play a key role not only as a primitive immune cell that cleans up after an infection but also both in connection with brain damage in general and with several other disorders of the central nervous system.
The accumulation of these microglia actually turned out to be a very common feature of most of the scrapie diseases such as mad cow disease and the equivalent in sheep and goats. So the microglia were closely related to the prions, and it was even suggested that microglia might be the trigger releasing these neurodegenerative prion diseases.
Marco Prinz is Director at the Institute of Neuropathology, Medical Center, University of Freiburg, Germany. © Britt Schilling
Much more central role
The microglia may even not be as boring as people thought. After 4 years in Zurich, Marco Prinz returned to Germany and became group leader at the Institute of Neuropathology, University of Göttingen. The future seemed bright, but convincing potential donors and research councils that studying inflammation of the brain is important was difficult.
“They said: he has done some good research, but he has no evidence for what he wants to do now. I got positive feedback, but rejection after rejection. You really have to be in the right place at the right time in research.”
2005 turned out to be the pivotal time. Neuroscience was experiencing a renaissance. With new laser scanning imaging techniques, confocal microscopy and new techniques for studying the brain in mice using green fluorescent proteins, for which Roger Tsien received the Nobel Prize in 2008, opportunities to study brain cells in great detail exploded.
Now we could suddenly follow the actual development of the cells and thus also see what factors trigger the development of microglia in the embryo, and we increasingly realized that microglia are not simply the first important immune barrier against pathogens and environmental changes. They seemed to play a much more central role in the brain.
Link to specific diseases
In 2007, Marco Prinz and his colleagues in Göttingen discovered that immune cells, which express high levels of chemokine receptor CCR2, develop when inflammation occurs in the brain’s microglia-like cells, such as in the autoimmune disease encephalomyelitis – the animal equivalent of multiple sclerosis.
Almost overnight, microglia suddenly went from being the dullest to the most exciting topic, and I had been working with microglia for more than 10 years, which gave me a huge advantage over many other researchers.
In 2008, Marco Prinz was offered a professorship at the University of Freiburg, where he became director of the Institute of Neuropathology at this very early stage in his career. He was also made responsible for neuropathological diagnostics at the University’s medical centre and the surrounding hospitals.
“This enabled us to better identify the links to specific diseases, including Parkinson’s, Alzheimer’s, Huntington’s and multiple sclerosis, and it soon became clear quite quickly that microglia have many functions in the brain – both during development and maturation and in regenerating brain cells.”
200 genetic links
Convinced of the central role of microglia, Marco Prinz now decided to focus his research on their origin and how they evolved. At the time, it was thought that monocytes (immune cells derived from bone marrow) – the immune system’s garbage collectors that clean up and destroy foreign organisms and dead cells – circulate in the bloodstream and penetrate the body’s various tissues and that these differ from the tissue’s own garbage collectors.
“If true, this fascinating concept could revolutionize treatment: simply manipulating the bone marrow would better combat diseases in various tissues.”
However, Marco Prinz and his colleagues discounted this theory and showed in a groundbreaking study that both the brain tissues’ own garbage collectors – the microglia and the cells in perivascular tissues, the sites around blood vessels – originated from similar stem cells in the fetal yolk sac.
10% of brain cells are plastic immune cells
The brain’s immune system is therefore off limits. The microglia and the brain’s other immune cells arise in the fetal state, and when a baby is born, the influx of immune cells from the periphery to the brain is shut off, just like to other central organs.
“The brain is somehow protected by immune privilege. The development of the immune system differs from that of the skin and the kidneys, for example. You can live without a kidney, but some organs have to have immune privilege, so evolution has found a way to protect these key organs, such as men’s testicles, women’s ovaries and then the brain.”
According to Marco Prinz, curing many of the numerous brain disorders requires learning more about the brain’s own immune system and especially learning to understand the origin and function of microglia and how to manipulate them.
“Most of the billions of cells in the brain are neurons and are not very plastic. However, the 10% that are microglia are very plastic, and in recent years we have found that about half of all genes associated with a higher risk of brain disorders are present in microglia. So perhaps we can cure diseases by manipulating the microglia. However, this is a huge puzzle we are assembling, and although each piece adds something new, there is still a long way to go.”
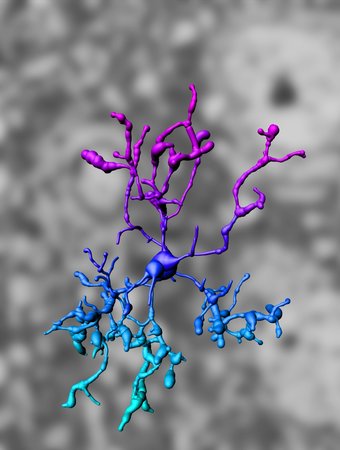
IMARIS-based three-dimensional reconstruction of a microglia cell (from Goldmann, Wieghofer et al. 2013).
Gut linked to the brain
In 2015, Marco Prinz and colleagues found a surprising piece of the puzzle when they revealed that the microorganisms in the human gut play a key role in regulating the maturation and activation of microglia.
This suggests that environmental factors may actually control the microglia and may prove to be key elements in understanding and managing brain health. In particular, microglia appear to be especially affected, since insufficient diversity and complexity of the microbiome can lead to defects in the maturation, differentiation and function of microglia.
The study indicated a key role for the communication between gut and brain in health and in disease. In a later study, in which they linked the composition of the microbiome to activation of special genes on microglia, the researchers showed that dysregulation can ultimately result in disease.
“These genes can be linked directly to the development of Alzheimer’s disease. Since then, we have carried out similar studies on Parkinson’s disease and multiple sclerosis with similar results, so we first try to understand how the sick person’s microbiome has changed and then we identify the factors linking the gut and microglia that make people sick and, ultimately, how to change the microbiome to affect and cure the disorder.”
Brain confetti party
In his efforts to understand the diverse role of microglia in the brain, Marco Prinz and his group recently became more and more aware that microglia, in addition to being the brain’s local garbage collectors, also help to create plasticity and structure in the brain. By combining single-cell RNA sequencing and mass spectrometry, they created a breakthrough in understanding the normal and diseased brain.
This enabled us to identify and characterize the gene expression in the cells and compare this with, for example, the development of tumours. The gene expression and plasticity of the microglia play a special role in this development. Whether the altered genetic expression is the cause and whether this changes because of the development of the tumour is still too early to say.
Researchers have also turned their attention to the microglia in connection with the scientific black box of nervous system and mental disorders. Today, we know that both autism and schizophrenia alter neuronal networks, and these previously boring cells are suspected once again.
“We have developed a confetti system to colour the microglia in mice red, yellow, green and blue – depending on whether they are expanding by creating daughter cells, contracting or remaining the same. This enables us to monitor the development of the cells in the normal state and during the development of various diseases.”
A real goldmine
Twenty-five years after Marco Prinz launched himself into boring brain cells, microglia are today considered key regulators of the central nervous system – both in health and disease.
“They seem to be involved in every form of brain pathology: in neurodegeneration such as Alzheimer’s disease, amyotrophic lateral sclerosis and Parkinson’s disease; in neuroinflammatory diseases such as meningitis and multiple sclerosis; in nervous system and mental disorders such as autism and schizophrenia; and even in pathological conditions.”
The microglia are thus not simply immune cells that clean up after infections that can damage the brain, including in autoimmune diseases such as Parkinson’s disease in which the immune system attacks itself. The cells also build and shape the environment in the neuronal networks. As Marco Prinz collects more and more information about microglia, they are increasingly proving to be a real gold mine.
“Many companies are trying to target these cells therapeutically. Today we know that, although Alzheimer’s, Huntington’s and Parkinson’s and multiple sclerosis are multifactorial and complicated diseases with more than 200 genetic mutations and risk factors for each disease, the microglia provide most of the genetic risk factors.”
Although Marco Prinz and colleagues dream of using their knowledge of the cells to understand and possibly cure the diseases, this remains complicated.
“The brain is probably the most complex thing in the universe. Developing new types of therapy can therefore easily turn out to be much more complicated than we expect, so our initial vision is to use the new knowledge to develop better diagnostics, whereas new microglia-based therapies are probably a little further in the future.”
A diagnostic alarm clock
According to Marco Prinz, a diagnostic method based on microglia may become a reality in the not-so-distant future. Currently, many brain disorders are diagnosed using small biopsies.
“When a biopsy is taken, people already have the disease, often already at an advanced stage. Microglia are ultimately the first cell to respond during almost any brain disease. Identification of some molecules, genes or markers that are really typical of microglia-specific disease will enable us to identify a disease much earlier and perhaps intervene with a treatment before they become too advanced.”
Microglia exhibit a cell-specific molecular signature, and thus, Marco Prinz can imagine basing a diagnostic method on deviations from this signature. Out-of-balance microglia may indicate early disease since many brain diseases take years or even decades to develop.
“Once the symptoms appear, it is often too late. One could imagine identifying some of the microglia-specific molecules in the future using magnetic resonance imaging or positron emission tomography among living patients at higher risk. This could act like an alarm clock in the brain that tells us, for a specific patient, that the microglia in a particular region are activated, and this can then inform our diagnosis and perhaps even our treatment in the long term,” concludes Marco Prinz.
