Novozymes Prize winner 2017 and Nobel Laureate 2020 Emmanuelle Charpentier has been one of the driving forces behind harnessing a bacterial immune system into the transformative CRISPR-Cas technology that has the potential to cure genetic diseases. Nevertheless, she asserts that she still has not achieved her goal, since bacteria comprise an inexhaustible resource of knowledge be exploited for further biotechnological and biomedical applications.
Bacteria can be compared to the characters of Dr. Jekyll and Mr. Hyde. Just when we think we know them, they surprise us completely. At their worst, bacteria can kill people. Conversely, at their best, bacterial populations such as those in our gut microbiome can improve our health. Emmanuelle Charpentier has dedicated her life to answering fundamental questions about physiological and regulatory processes in bacteria.
This mission led her to investigate a bacterial immune system called CRISPR-Cas9 and to develop this system into a genome engineering technology.
"Bacteria have always fascinated me. The incredible diversity of the bacterial world testifies to how evolution works in practice. There are countless types – those that make us sick and those that help us. Research on bacteria is invaluable because it gives us not only strategies to combat multidrug-resistant bacteria but also tools such as CRISPR-Cas9 for biotechnological and medical applications,” explains Emmanuelle Charpentier," Director of the Department of Regulation in Infection Biology at the Max Planck Institute for Infection Biology in Berlin, Germany.
Emmanuelle Charpentier has led a diverse and highly mobile research career, and has experienced both the positive and negative aspects of such a varied path. She has strived for 20 years to be recognized by a research field that didn’t place much value on moves between universities and research environments.
"I have always considered it important to follow my intuition. From the beginning, I believed that I – like many other professors – would eventually find a new niche and open a new field of research for myself and others. However, it slowly dawned on me that I was not looking for a specific theme, and that I enjoyed the freedom of exploring different fields. Throughout my career, the red thread has been working at the crossroads between basic, clinical and applied research."
"My research should be novel, and it should make a difference."
The discovery of CRISPR-Cas9 genome editing, and the recognition that came with it, have encouraged Emmanuelle Charpentier to further explore the world of bacteria for additional potential breakthroughs.
Face-to-face with antibiotic resistance
The hunt to reveal the secrets of bacteria began at the Pasteur Institute in Paris. Emmanuelle Charpentier, a young PhD student working in the laboratory of Patrice Courvalin, started to work on antibiotic resistance, which was emerging as a major worldwide health threat. At that time most antibiotics were still effective, so the discovery of multidrug-resistant Listeria bacteria came as a huge shock. These bacteria can cause meningitis and blood poisoning among infants and older people.
"It certainly makes you want to make a difference when, as a young person, you find yourself looking at the test results from an 84-year-old man with meningoencephalitis whose life was being threatened by multidrug-resistant bacteria. This was the moment that piqued my interest in the physiology of bacteria and clinical research. How did the resistance occur, and how could we develop treatment?"
Charpentier and Courvalin showed that the transfer of genes that confer resistance to antibiotics occurs not only between bacteria of the same species but also between bacteria of different species. For instance, Listeria could inherit resistance genes from bacteria in the human gut. In this way, bacteria can rapidly pick up each other’s tricks for combating specific types of antibiotics.
"Although I had already become interested in bacteria, clinical research and developing treatments, I understood that I had to go out into the world and forge my own research field. I figured that if I went to the United States and became an expert in streptococci, I would be able to return and start my own research group within this field."
The first major breakthrough
In 1996, Charpentier relocated to the laboratory of Elaine Tuomanen at the Rockefeller University in New York and then to St. Jude Children's Research Hospital in Memphis, Tennessee with the purpose of studying streptococci, which are an even greater health threat than Listeria. But when she arrived, stark differences in the research environment in the United States gave Charpentier cause for thought.
"Witnessing the massive biotechnology efforts that gripped the United States from the mid-1990s onwards was incredibly inspiring. As a medical microbiologist coming from a more conservative European research tradition, it was incredible to see how the research environment in the United States built bridges between the academic world and the pharmaceutical industry."
Charpentier’s research also scaled new heights under Elaine Tuomanen, some of whose research focused on life-threatening streptococcal diseases among children. The antibiotic vancomycin had been the last bastion of defence against these bacteria, and researchers knew that a catastrophe would be inevitable when this antibiotic ceased to work. Their critical discovery of the molecular mechanism in the streptococci that caused the vancomycin resistance was published in Nature, one of the world’s most prominent journals.
"I sought out and was fortunate to find the best mentors. Each one has greatly influenced my career. The combination of basic and applied research has also driven me forward. Finally, it has been important for me to constantly re-evaluate my research, to be open for changes in direction and hopefully make a difference in my field of research."
Before her next move, Charpentier and her fellow researchers, especially Rodger Novak, succeeded in revealing important details about the mechanisms of antibiotics. What they discovered was that antibiotics such as penicillin do not directly kill cells but first initiate a process of apoptosis in the bacteria that ultimately leads to the bacteria dissolving their own cell membranes and dying.
A decision to stay in academia
Although Emmanuelle Charpentier had actually planned to return to Europe after her stay in the United States, she extended her visit with positions at the New York University Langone Medical Center and the Skirball Institute of Biomolecular Medicine in New York City. However, no long-term positions were waiting for her when she returned to Europe in 2002.
"It was as if they considered it a fault that my research career had not focused on a specific theme. I instead had found a way of carrying out research that integrated basic and medical research and spanned studies of bacteria and eukaryotes. I had become used to the research freedom in the United States. In those days, the research environment in Europe was much more old-fashioned."
Although at one point Charpentier considered applying for a job in biotechnology, she ended up being recruited as a junior group leader at the University of Vienna, where she had the freedom to develop her own research group. For a variety of reasons, she became interested in the emerging regulatory role of RNA molecules in bacteria and set out to discover and characterize novel RNAs in streptococci.
"The research clearly showed that RNA molecules have important regulatory functions in bacteria. Researchers had already discovered multiple mechanisms of regulation of gene expression by RNA molecules. For example, it had been shown that RNA can act at the level of transcription and translation by using anti-sense mechanisms or by binding to protein effectors. Very little was known about RNA biology in the human pathogen Streptococcus pyogenes, and I thought there was a good chance that novel mechanisms might be found. It certainly seemed to be an exciting field around which to build a laboratory."
Detect and destroy
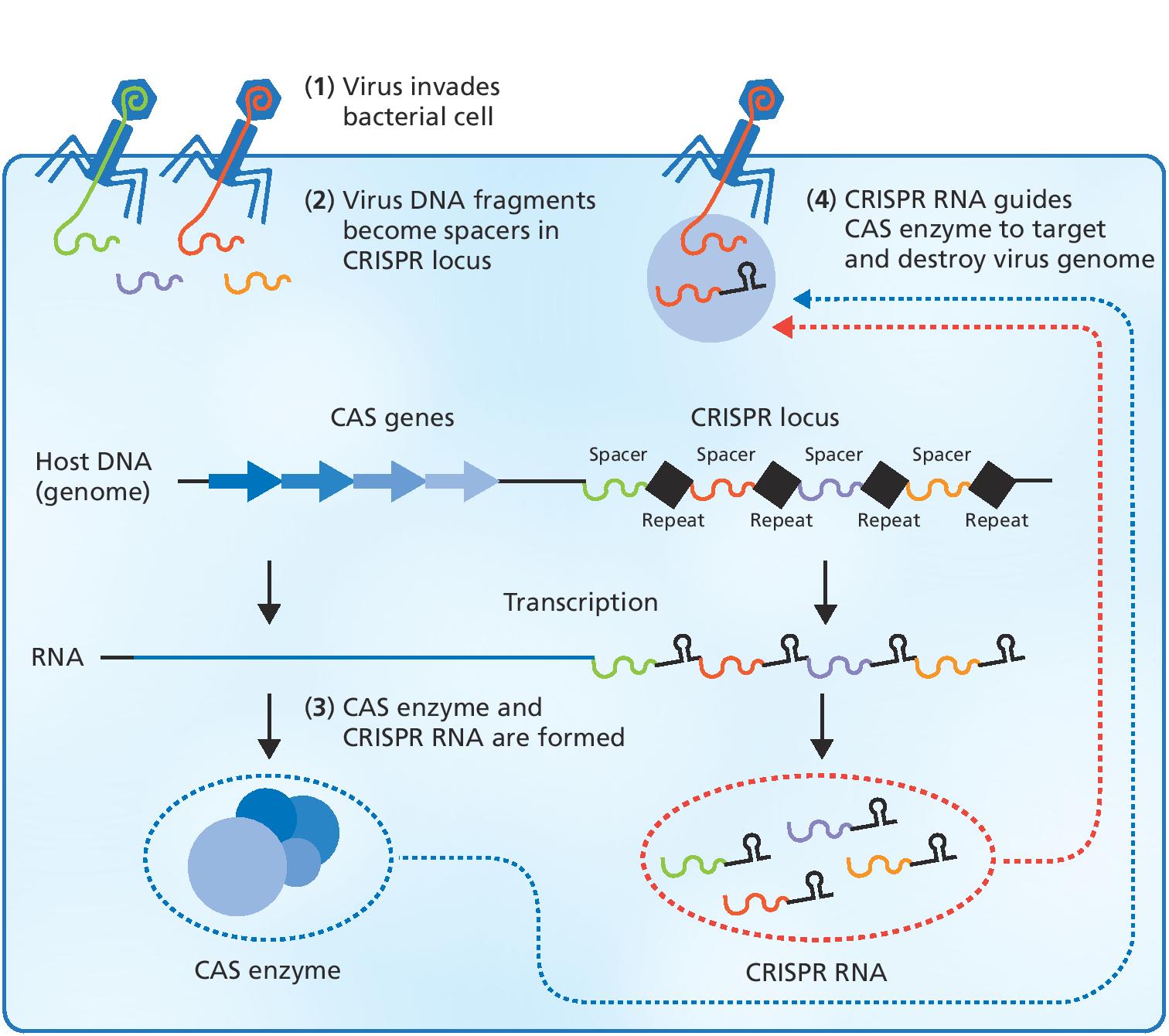
Charpentier’s group subsequently discovered that an RNA molecule plays an important role in the ability of streptococci to invade tissue. During this work on RNA molecules, Charpentier came across several studies on a bacterial immune system called CRISPR, where bacteria cut small fragments of DNA from invading viruses and then incorporate them into their genome so they can remember the DNA sequence again when they are attacked. You could call it a sort of genetic vaccination card.
What really captured Charpentier’s attention, however, was that bacteria make small RNA copies of the incorporated viral DNA sequences. If these RNA molecules recognize and bind to the corresponding viral DNA sequence upon subsequent infection, the cell knows that this is an invasive virus that must be destroyed.
During this time, Charpentier had started a new laboratory at the Laboratory for Molecular Infection Medicine Sweden in Umeå, Sweden. In 2006, she and her team performed a bioinformatics screen that resulted in the identification of numerous novel small RNA species in S. pyogenes, and this gave the researchers a surprise.
"We also found the small CRISPR RNA (crRNA) molecules with sequences that were identical to the viruses the bacteria had encountered along the way. However, what astonished us was the finding of an abundant RNA species containing a stretch of sequence with remarkable similarity to the repeats of crRNA."
Like a smartphone
Charpentier and her colleagues had discovered the last piece of the puzzle in the CRISPR-Cas9 system – the socalled trans-activating RNA, or tracrRNA. As reported in a study published with Jennifer Doudna and other colleagues, the tool that resulted when crRNA and Cas9 protein were mixed with the new RNA fragments in a test tube was a fully functional DNA cleavage system.
Not surprisingly, Charpentier’s discovery of tracrRNA triggered an explosion of research in the CRISPR field.
"We discovered with Jennifer Doudna and our teams that by simply changing the sequence of the crRNA we could reprogramme the Cas9 enzyme to cut in another location. This is an incredibly beautiful design, and nearly as elegant as a smartphone – simple and user-friendly, but nevertheless both versatile and sophisticated. When we saw this, we knew that we had discovered a gene technology tool that matched anything we had previously seen."
CRISPR-Cas became the revolution that Charpentier had envisioned. After these groundbreaking studies, several groups showed that the system not only functioned in bacteria and in the text tube but also in diverse cell types in plants, animals and humans. The system could thereby be used to repair the human genome by getting CRISPR-Cas9 to precisely cut out incorrect gene sequences and replace them with new sequences.
Talk of the town
Overnight, CRISPR-Cas9 grew from a specialist field of scientific research to a topic in the mainstream media. Excitement grew around the potential application of CRISPR-Cas9 for curing genetic diseases. Charpentier and her colleagues have received numerous scientific accolades, and they were listed in Time Magazine’s Top 100 most influential people in the world.
"As a young group leader, I focused on building my research programme with the hope of discovering new mechanisms that could be relevant for the scientific community. I knew when I received the first scientific prize for CRISPR-Cas9 in 2014 that it would not be the last one. One of my friends said to me: ‘You should accept, because if you don’t, there will almost certainly be someone else in the wings ready to claim the honour’."
Since 2014, Charpentier has received more than 40 major scientific prizes and distinctions, including the prestigious Warren Alpert Foundation Prize, the Gruber Foundation International Prize and the Breakthrough Prize in Life Sciences. It would not be surprising if she would be considered for a Nobel Prize at some point.
"An award such as the Novozymes Prize really means a great deal to me because it is people from another research field – biotechnology – that have examined my work and said that it is very important for their work. I see the Prize primarily as a prize for my field – microbiology – and as recognition of the importance of this research."
Beyond CRISPR
The significance of microbiology and the search for new ways to apply knowledge of bacterial systems to benefit people has filled much of Charpentier’s daily activities in recent years. CRISPR has dominated the attention the last 6 years, and the worry that this would swallow up everything has caused her to pause for thought. With receiving up to 20 prizes and distinctions annually, and the associated interviews and travel, staying focused is important.
"There are many distractions that can make me lose focus on what I really believe to be important – science – and I am concerned that I will be snared in my own net and will forever be known solely as the woman who discovered CRISPR-Cas9. However, I feel that there is much more that I want to accomplish, which is why I am doing my best to integrate my responsibilities and duties associated with CRISPR-Cas9 into my scientific life."
Since 2015 Emmanuelle Charpentier has been the Director of the Department of Regulation in Infection Biology at the Max Planck Institute for Infection Biology in Berlin. Here she has expanded her research program, which currently comprises five research fields.
"We are still working on the biology of CRISPR-Cas. The greatest progress we made was the work published in Nature last year, when we reported our discovery of an even simpler system from the bacterium Francisella novicida. In this system, the Cpf1 enzyme not only activates crRNAs, but also targets the viral DNA. This means that we end up with an even simpler and more elegant system."
A free electron
Emmanuelle Charpentier feels liberated at this stage of her career, or as she puts it, “like a free electron” that can move freely through the bacterial treasure trove in places she believes are important.
"We see bacteria as toolmakers. Bacteria encode a large variety of enzymes that have revolutionized biology. These enzymes have enabled us to read whole genomes, including the human genome. They also produce the enzymes that can cleave and ligate DNA molecules, which have enabled cloning. With the CRISPR-Cas9 system, they have supplied us with a powerful tool to perform gene surgery to repair genetic mutations."
The next major challenge is to deliver the CRISPR-Cas9 system into the right tissues so the repair can take place in exactly the right places, for example, the brain, the heart or any other tissue in the body. For Emmanuelle Charpentier, however, there is much interesting work to be done beyond CRISPR-Cas9.
"Apart from our continuing studies of CRISPR, we also study, for example, the small regulatory RNAs that interfere with bacterial pathogenicity and the mechanisms of bacterial recognition by immune cells. Continuing basic science on bacteria will reveal new types of enzymes that may also be very useful for genome editing. And with the growing antibiotic resistance, there is certainly also a need to better understand infectious diseases caused by bacteria."
Most important for Charpentier, however, is her continuing work to train young scientists to ensure the future of science. Major donations from the Kempe Foundation and the Wallenberg Foundations have given her the opportunity to train more young researchers.
"Working with young students is the most inspiring part of the job and extremely important for the scientific community. I try to teach what has been most important for my career and my research: to follow your gut feeling and to believe in your ideas but to keep an open mind to new ideas. Without this open mind, I would never have found what we discovered with CRISPR-Cas9."
The Nobel Prize in Chemistry 2020 has been awarded to Emmanuelle Charpentier and Jennifer A. Doudna. The 2017 Novozymes Prize was awarded to Emmanuelle Charpentier, Director, Department of Regulation in Infection Biology at the Max Planck Institute for Infection Biology in Berlin, Germany, and Virginijus Siksnys, Professor and Head of the Department of Protein–Nucleic acids Interactions at the Institute of Biotechnology of Vilnius University in Lithuania.
